Has the paradigm of botulinum toxin A changed? Efficacy and side effects of injections into the bladder wall for OAB
In science and philosophy, a paradigm is a distinct set of concepts or thought patterns, including theories, research methods, etc., which constitute legitimate contributions to a field. The use of botulinum toxin certainly constitutes a legitimate contribution to the therapeutic approach of overactive bladder (OAB) patients.
Lower urinary tract symptoms OAB is defined by the International Continence Society (ICS), as ‘urinary urgency, usually accompanied by increased daytime frequency and/or nocturia, with urinary incontinence (OAB-wet) or without (OAB-dry), in the absence of urinary tract infection or other detectable disease.(1)’ This set of lower urinary tract symptoms (LUTS), which affects 10.7% of the worldwide population(2), usually leads to a great negative impact on the quality of life of a significant part of the population(3). For over 20 years – since the term “overactive bladder syndrome” was first introduced to the urological community community - more than 5,000 articles have been published in specialty journals.
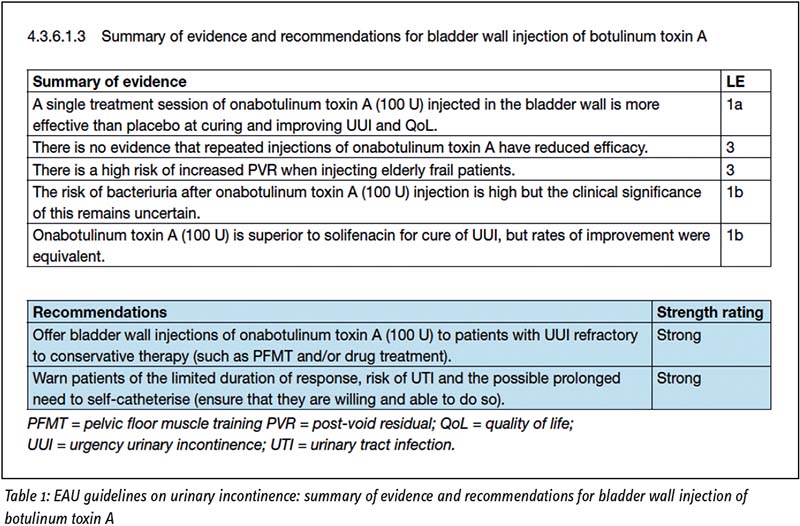
According to the EAU guidelines on urinary incontinence, if dietary and fluid recommendations fail, patients should be offered either oral antimuscarinics and/or β-3 agonists. When medications fail, patients are considered for minimally invasive techniques such as intradetrusor botulinum toxin A (BTX-A), posterior tibial nerve stimulation (PTNS), or sacral neuromodulation (SNM). Despite recent advances in the medical treatment of OAB, non-compliance to oral antimuscarinics is still high(4). According to a recently published study, the proportion of patients adherent to OAB medical therapy at 1 year varies between 15% and 44%.
Onabotulinum toxin A Onabotulinum toxin A (OnaBoNT-A) accounts for the largest number of scientific studies and was approved for the treatment of urgency urinary incontinence by the Food and Drug Administration (FDA) in 2013. Other botulinum toxins, such as abobotulinum toxin A and incobotulinum toxin A, were less frequently studied for bladder indication, so that their efficacy and safety are not well known. In addition, their use is not provided on label for this indication(5).
The efficacy and side effects of the treatment with OnaBoNT-A depend on the dose used. The recommended dose for patients with idiopathic OAB is 100 U. The use of 150 U results in a discrete improvement of efficacy, but higher risks of urinary retention. Most studies evaluated the botulinum toxin injection into the body of the bladder, sparing the bladder trigone. Different protocols were used, including injections into 10-20 bladder sites with such dilution to inject 0.5-1.0 mL per site. OnaBoNT-A injection should be performed deeply into the detrusor. Submucosal injection was less often evaluated and its results are not well known. OnaBoNT-A injection can be performed with local/ general anaesthesia, sedation or spinal block.
The need for reinjections over time it is critical that patients are carefully educated about the risks and accept the possibility that they need bladder intermittent catheterisation (IC) in case of urinary retention. Patients should also be informed that the effects of the treatment diminish over time (mean duration of effects around 9 months) and that the vast majority will need reinjections.
There are several factors which should be carefully reviewed to explain why the paradigm of botulinum toxin A injection in the bladder wall for OAB may change soon:
Despite being effective, the adherence to BTX-A is low in the long run Although intradetrusor injection is an established treatment for OAB patients, most studies have evaluated efficacy and compliance to this treatment in a predominantly female population at short-term follow-up. Mohee et al followed OAB patients after their first BTX-A injection and demonstrated that almost two-thirds of patients (61.3%) had discontinued therapy at 36 months, with a 63.8% discontinuation rate at 60 months. The main reasons for discontinuation were tolerability issues(6.)
Rahnama’i et al7 evaluated the long-term compliance of BTX-A in a heterogeneous group of male patients who underwent intradetrusor injections between 2004 and 2010 at a single centre. The mean follow-up was almost 6 years. 24 patients presented with idiopathic OAB (mean age 67 years). Only 5 (21%) persistent on BTX-A therapy in the long run. This is less than the rates reported in the literature for idiopathic OAB treatment in women. Most important factors for poor compliance were insufficient effect (n = 8) and adverse events (n = 10).
Urinary retention has been reported in up to 30% of patients According to a systematic review of the literature8, the need for IC at doses of 100 units (the on-label dosage for patients with idiopathic OAB) ranged from 6.9–30%9,10. It should be noted, however, that the indication of catheterisation varied among the studies. Such differences between the definitions for urinary retention and the need for IC render comparison among studies difficult. Regardless, retention is transitory and dose-dependent.
More recently, the 2-year outcomes of the Rosetta trial have been published11. This was a multicentre, open-label, randomised extension trial (February 2012-July 2016) at 9 US medical centres involving 386 women with ≥ 6 urgency urinary incontinence episodes (UUIE) over 3 days, which were inadequately managed by oral medications. Participants were clinical responders to treatment: ≥ 50% reduction in UUIE after sacral neuromodulation implant or 1 month post BTX-A injections. Over 24 months, 72% (115/159) of the BTX-A participants requested a second injection (median interval between first and second injection was 350 days). In this group, 101/115 (88%) had 200 U of which 6 (6%) required IC. Patients with a postvoid residual of more than 300 mL or more than 200 mL and symptoms of incomplete voiding were instructed to perform IC after treatment. Per protocol, participants requiring IC for > 6 weeks after initial BTX were dose-reduced. Specifically, 14/115 (12%) were dose-reduced from 200 U to 100 U, and 3/14 (21%) still required IC after 100 U. Median CIC duration across the nine participants was 29 days (interquartile range = 17–56 days).
There are other effective options as third-line therapies for OAB Non-invasive and invasive electrostimulation techniques have been extensively studied in the treatment of lower urinary tract dysfunctions, including OAB.
Percutaneous tibial nerve stimulation (PTNS) is a minimally invasive office-based procedure, which has been shown to be safe and effective in treating OAB symptoms12. The patients must be able to make frequent hospital/clinic visits to receive the therapy, making it time-consuming for the patient and costly for both the patient and healthcare system. Most PTNS protocols suggest 12-weekly treatments followed by less frequent treatments to sustain OAB symptomatic improvement. Heesakkers et al13 have recently published a prospective multicentre study assessing the safety and performance of the RENOVA system in the treatment of patients with OAB with or without urgency incontinence Thirty-four of the 36 implanted subjects completed the study. At six-month follow-up, 71% experienced clinical improvement (based on bladder diaries).
Sacral neuromodulation (SNM) is another established third-line treatment for refractory OAB. SNM electrically stimulates somatic afferent nerves in a sacral spinal root (usually S3) and sends signals to the central nervous system that may restore normal bladder function. Activation of somatic afferent nerves inhibits bladder sensory pathways and detrusor overactivity14. Until recently, Interstim® (Medtronic, Minneapolis, MN (US)) was the sole device on the market used to deliver sacral neuromodulation with demonstrable OAB therapeutic success rates as high as 83%15. Main downsides of SNM therapy include device-relatedadverse events, lack of magnetic resonance imaging (MRI) compatibility and need for surgical reintervention. Studies show that by 36 months of SNS therapy 11% of subjects require battery replacement due to depletion16. Therefore, development of new SNM technologies has concentrated on providing improvements to decrease device-related adverse events and surgical re-intervention.
New SNM devices / MRI compatibility Axonics Modulation Technologies has recently developed a new SNM device for the treatment of OAB. The device was approved in 2016 for the treatment of OAB in Europe and Canada and consists of a small volume rechargeable neurostimulator (60% smaller than the currently available Medtronic device) and a tined lead with four electrodes percutaneously inserted through the sacrum. The tined lead is subsequently connected to a pulse generator implanted in the upper buttock area. The implantable pulse generator battery is rechargeable with a 15-year lifetime in the body, which may obviate frequent IPG replacements. In addition, the device is current controlled: output voltage is automatically adjusted based on tissue impedance. According to the manufacturer, this may provide more consistent therapy. An important consideration is the possibility of future MRI studies for the patient. The FDA approved the Axonics r-SNM System for full-body 1.5 Tesla MRI scans in September 2019. This differs from the recommendation for the standard Interstim-2 device, which is only approved for 1.5 T head MRI.
Medtronic has received CE Mark approval for its InterStim Micro neurostimulator and InterStim SureScan MRI leads in January 2020. By making full-body MRI scans possible, the firm has increased accessibility to sacral neuromodulation (SNM) therapy for European patients. InterStim Micro is a rechargeable device, which offers sacral neuromodulation therapy for the treatment of overactive bladder (OAB), faecal incontinence (FI) and non-obstructive urinary retention. InterStim Micro is said to be 80% smaller than the already existing recharge-free InterStim II neurostimulator. The SureScan leads, which will be used in both the InterStim Micro system and in future implants of the existing recharge-free InterStim II, are designed to allow for full-body 1.5 and 3 Tesla MRI-conditional scans. Medtronic claims that it is the only firm in Europe to provide patients with a choice between rechargeable and recharge-free systems, which are both full-body MRI-conditional17.
New advances in neuromodulation techniques may impact on patients’ decision about third-line therapeutic approaches to OAB.
Despite being a minimally invasive procedure, the need for bladder wall injections to deliver the medication is a barrier Bladder application of BTX-A requires a cystoscopic procedure with intradetrusor needle injection into 20 to 30 sites. The patient is under local anaesthesia or sedation. The avoidance of intradetrusor BoNT needle injections may potentially decrease the risk of complications, such as haematuria, pain, large PVR and UTI. Because of the limited permeability of the bladder wall, OnaBoNT, which is a large molecule (approximately 150 kDa), has limited access to the submucosal nerve plexus when not injected with a needle.
Recently Chuang et al demonstrated that bladder uptake of BoNT improved when the toxin was formulated with liposomes in a in an overactive bladder model in rats bladder model in rats18. Liposomes (lipid vesicles) have been extensively studied as a drug delivery platform for anticancer drugs, and several such products have received FDA approval. Liposomes have been previously studied as toxin carriers to enhance efficacy at lower doses. They rely on vesicle endocytosis for intravesical drug delivery19.
A multicentre, placebo-controlled study to assess the safety and efficacy of onaBoNT-A complexed with liposomes was performed in men and women with OAB20. This treatment successfully reduced urinary frequency and urgency; however, the treatment did not reduce UUI episodes. Furthermore, onaBoNT-A complexed with liposomes did not result in urinary retention. Risk of UTI was similar between placebo and treatment arms. We may conclude that instillation of onaBoNT-A complexed with liposomes successfully impairs afferent neurotransmission but not the efferent neurotransmission.
What the future holds for BTX-A is unknown. Feasible advances in clinical practice are still eagerly awaited by the international urological community and by patients suffering from OAB symptoms.
References
Haylen BT, de Ridder D, Freeman RM, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk D, Sand PK, Schaer GK An International Urogynecological Association (IUGA) / International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn, 2010,29:4-20; International Urogynecology J, 2010,21:5-26
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108:1132.1138.
Int Braz J Urol. 2016 Mar-Apr;42(2):188-98. doi: 10.1590/ S1677-5538.IBJU.2015.0365. Overactive bladder - 18 years - Part I. Truzzi JC1, Gomes CM2, Bezerra CA3, Plata IM
Campos J5, Garrido GL6, et al. 4. Real-world persistence and adherence to oral antimuscarinics and mirabegron in patients with overactive bladder (OAB): a systematic literature review. Yeowell G, Smith P, Nazir J, Hakimi Z, Siddiqui E, Fatoye F. BMJ Open. 2018 Nov 21;8(11):e021889. doi: 10.1136/ bmjopen-2018-021889.
Int Braz J Urol. 2016 Mar-Apr;42(2):188-98. doi: 10.1590/ S1677-5538.IBJU.2015.0365. Overactive bladder - 18 years - Part I. Truzzi JC1, Gomes CM2, Bezerra CA3, Plata IM4, Campos J5, Garrido GL6, et al. 6. BJU Int. 2013 Jan;111(1):106-13. doi: 10.1111/j.1464410X.2012.11282.x. Epub 2012 Jun
Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). Mohee A1, Khan A, Harris N, Eardley I.
Due to space constraints, the entire reference list can be made available to interested readers upon request by sending an email to: communications@uroweb.org.
Prof. Marcio Averbeck Member, ICS Standardisation Steering Committee Moinhos de Vento Hospital Porto Alegre (BR)